Directions
We know that the number of protons defines each element, but what about the neutrons and electrons? Do they matter?
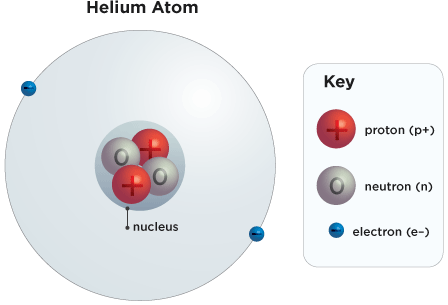
Let’s look at electrons first. Electrons are the tiny particles that fly around the nucleus. Electrons are negatively charged (like the negative end of a magnet) and they balance out the positively charged protons. In a perfectly balanced atom, the number of electrons always equals the number of protons.
Here are a few balanced atoms. Count the electrons and drag and drop the element names to match each element with its atomic diagram.
