Directions
Let’s take a closer look at chemical elements. If we zoom in real close, we’ll see that all chemical elements are made up of tiny particles called atoms. You may have seen an atom drawn like this:
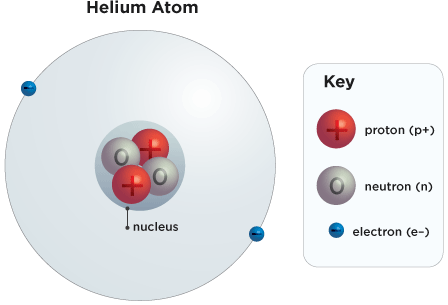
This is a diagram of a helium atom: 2 electrons (blue) circling a nucleus of 2 protons (red) and 2 neutrons (gray).
Protons are positively charged particles (like the positive end of a magnet). All atoms have protons, electrons and neutrons, but what makes each element unique is the number of protons. For instance, a helium atom has 2 protons. If you and give it another proton you get a different element altogether: Lithium, a metallic element with 3 protons.
The number of protons in an atom is known as the atomic number. So the element helium has the atomic number 2, because it always has 2 protons.
Count the protons and drag and drop the element names to match each element with its atomic diagram.
